
Valency, atomic number and symbol of next 30 elements in periodic table Brainly.in
Learn the concepts of Class 9 Chemistry Structure of the Atom with Videos and Stories. List the rules of writing the electronic configuration. Explain how electronic configuration determines the valency and in turn the reactivity of the elements. Join / Login > 9th > Chemistry. Valency Chart. 3 mins read. Revise with Concepts. How are.
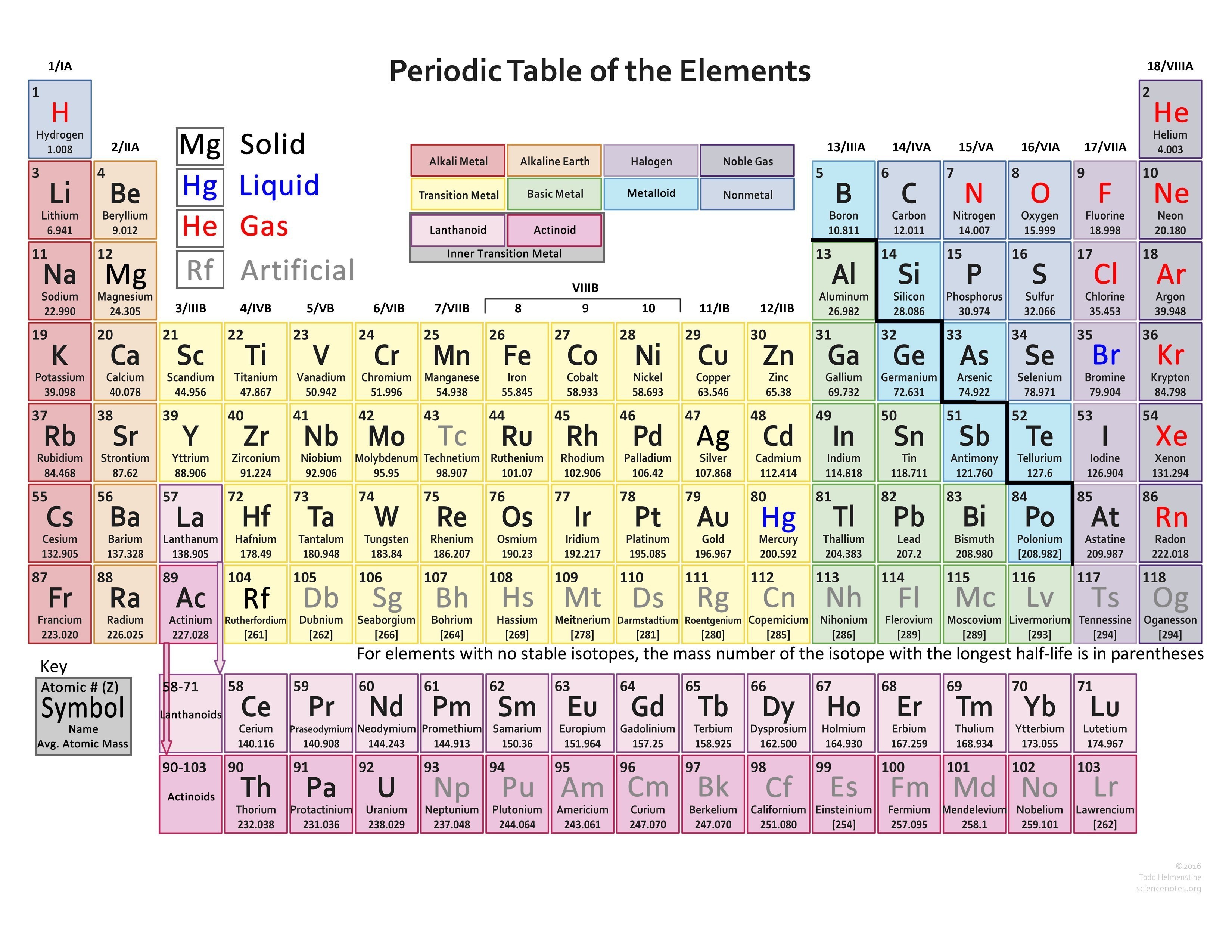
Find Various Types of Valency of Elements Valencies of 118 Elements
Step 1: The electronic configuration is 2 , 8 , 5 . Step 2: It has 5 valence electrons. Step 3: To attain a complete octet, atom needs to lose 5 electrons, so its valency is 5 . Where did he go wrong and why? Choose 1 answer: Step 3 is wrong. Valency cannot be 5 because it is difficult for an atom to lose 5 electrons. A Step 3 is wrong.

valency table for class 9 Brainly.in
Element Valency PDF. The outer shell of a fluorine atom contains 7 electrons. This means it has one less electron than needed to complete the shell. This gives fluorine a -1 valence. This element Valency PDF is a downloadable version of the Valences of the Elements table. As in the table, the most common valences are in BOLD text where values.
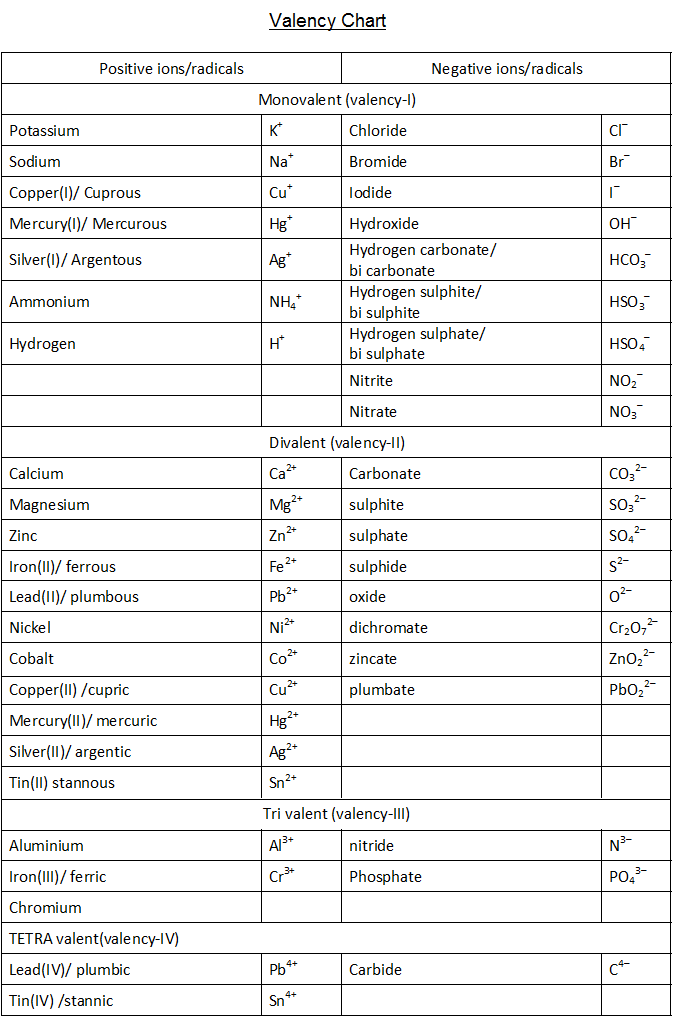
VALENCY CHART
Solution The combining power or the combining capacity of an element is called its valency. The number of atoms of other elements with which one atom of an element combines is decided by the valency of that element.
Valency Chart Science Notes Teachmint
CBSE Study Material Textbook Solutions CBSE Notes An Introduction to Valency Chart Valency can be defined as combining the power of an element or radical. The valency chart consists of the list of valencies of the element. We know the chemical formula of salt and water is NaCl and H2O respectively.

ICSE Class 9 Chemistry Additional Charts Reference Valency Chart PDF
Valency is the measure of the combining capacity of atoms or molecules. Therefore, it is the capacity of an atom of a single element to react and combine with particular numbers of atoms of another element. Browse more Topics under Structure Of Atom Introduction: Structure of Atom Atomic Number Bohr's Model of Atom Charged Particles in Matter

Class 9 Periodic Table Of Elements With Atomic Mass And Valency Periodic Table Timeline
The valence electron of an atom take part in a chemical reaction because they have more energy than all the inner electrons. For Example: (1) Sodium (Z=11) The electronic configuration of sodium is K L M 2 8 1

What is Valency Explain with Example Valency Definition Trick to Learn Valency of Elements
The valency chart is a table that shows the maximum number of bonds that an atom can form with other atoms. The chart is arranged so that each row corresponds to an element, and each column corresponds to a valence level.
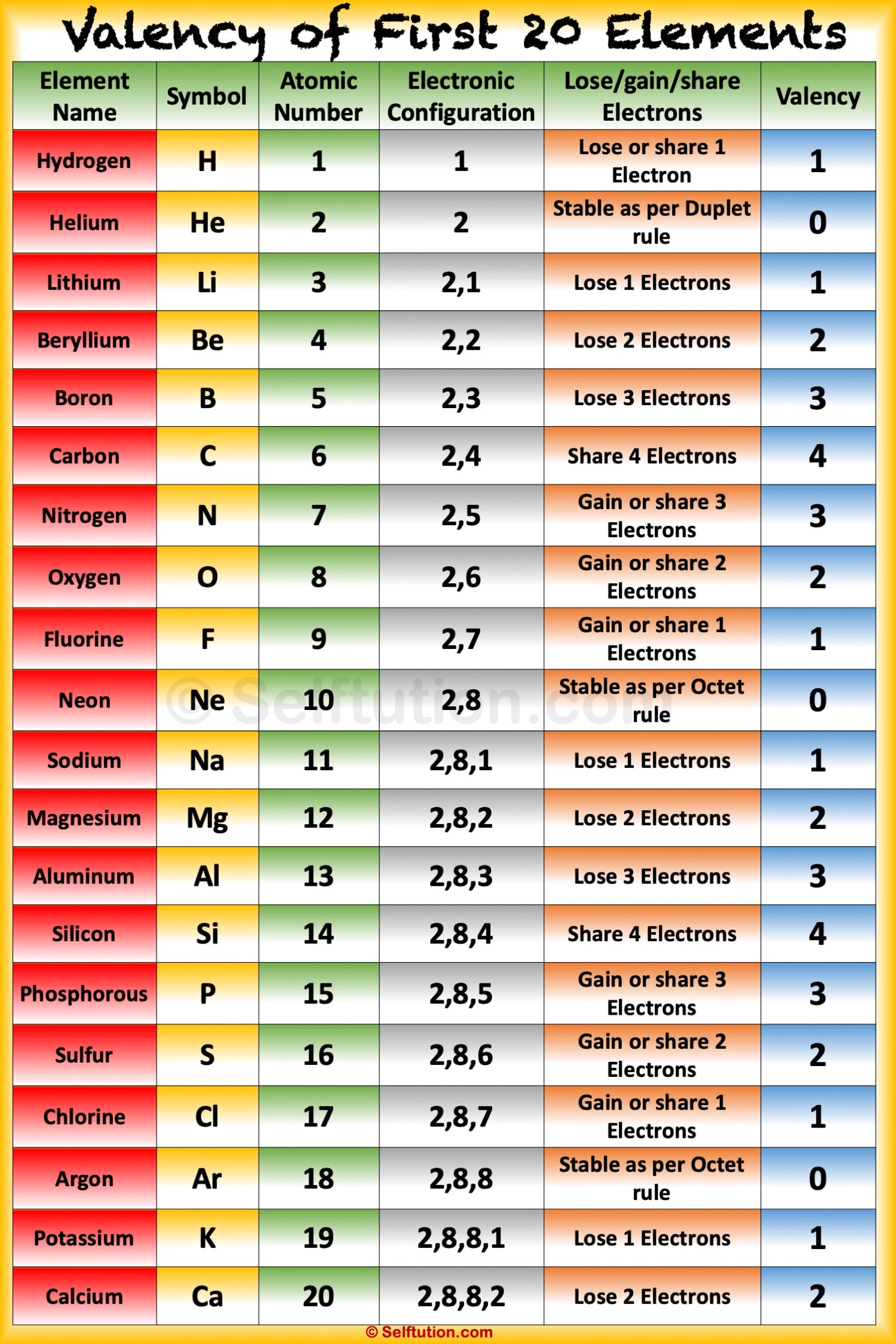
Valency and Variable Valency Valence Shell and Electrons » Selftution
A Valency Chart is intended for determining the amount of chemical bonds that a specific element can make with different elements. The valency table is a list of the element's valencies. Based on the element's valence electrons, those are the outermost electrons in charge of bonding, it describes how an element can form bonds.

valency table for class 9 Brainly.in
Valency Chart is a representation of the valency of different elements in the modern periodic table. Valency is the capacity of an element to combine with other elements. Valency refers to the number of electrons gained or lost to attain a stable electron configuration .

valency table Scribd india
The valency of an element is a measure of its combining capacity and can be defined as the number of electrons that must be lost or gained by an atom to obtain a stable electron configuration. What does the term 'Oxidation State' mean? The oxidation state of an atom is the number of electrons lost or gained by it.

Valency of an element, structure of the atom, class 9 science, chemistry, valency YouTube
Valency or Valence of an element is a measure of an atom's ability to combine with other atoms to create molecules or chemical compounds. The characteristics of an element that indicate how many more atoms can join one of its atoms in a covalent bond are known as valence, or valency, in chemistry.

Periodic Table Of Elements With Atomic Mass And Valency Bruin Blog
Notes of IX B, Science valency chart.pdf - Study Material. Notes of IX B, Science valency chart.pdf - Study Material. Dashboard Login Login Feedback. Logout. class-9th. Science. 0 Likes. 2 Views. Copied to clipboard T. Tr. Vanessa. Jan 14, 2022. Test. Why Do We Fall ILL class-9th. Science. 0 Likes. 7 Views. Copied to clipboard T.
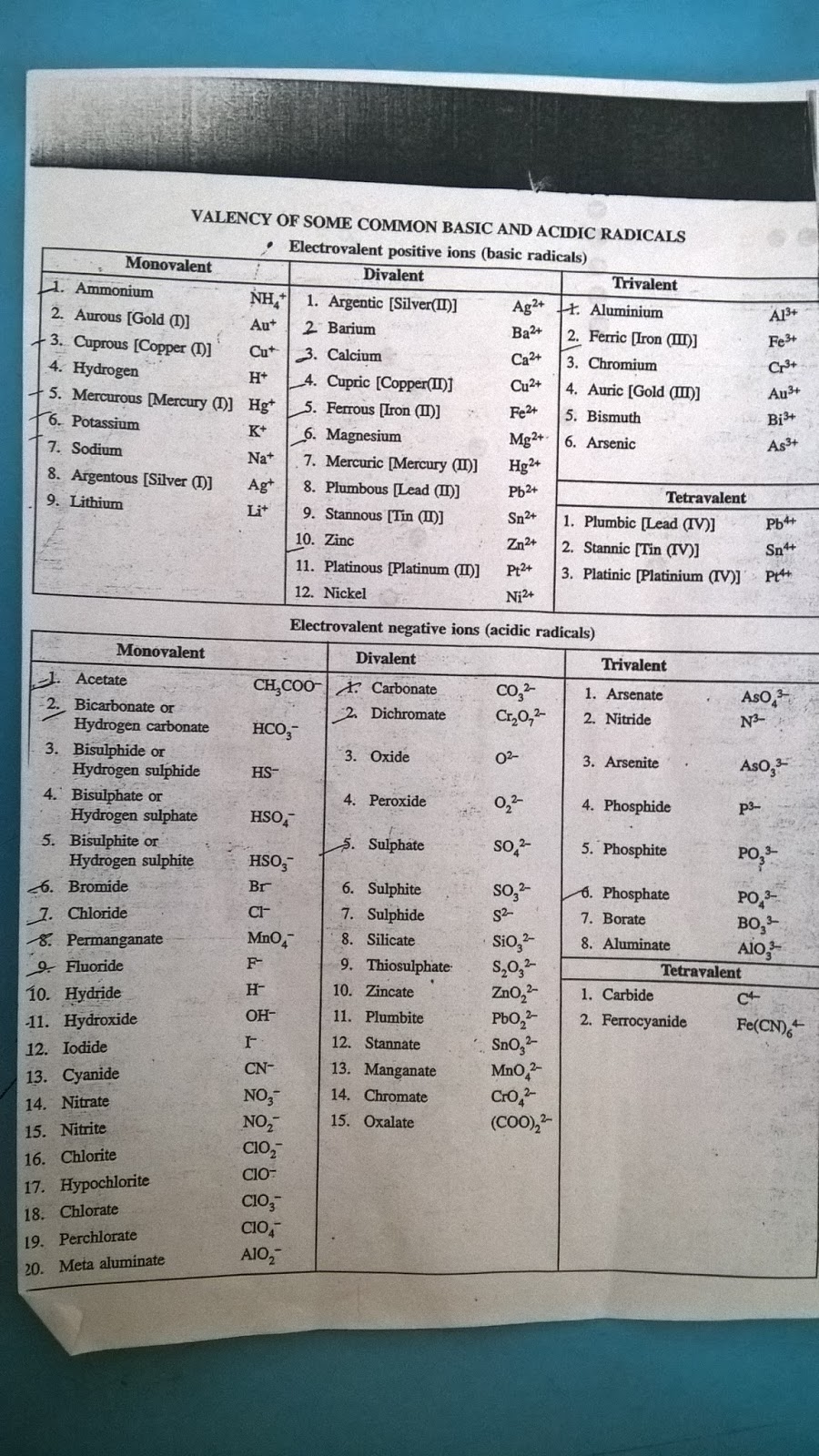
Valency Chart
Structure of Atom Valency What Is Valency? Define Valency The combining capacity of an atom is known as its valency. The number of bonds that an atom can form as part of a compound is expressed by the valency of the element. We all know how electrons in an atom are arranged in shells/orbitals.

Valency
The number of electrons that should be lost or gained to achieve this stable configuration is known as valency . Learn in your speed, with individual attention - Teachoo Maths 1-on-1 Class Book a free demo Next: What is Atomicity? Important → Ask a doubt Class 9 Chapter 3 Class 9 - Atoms And Molecules Tired of ads?
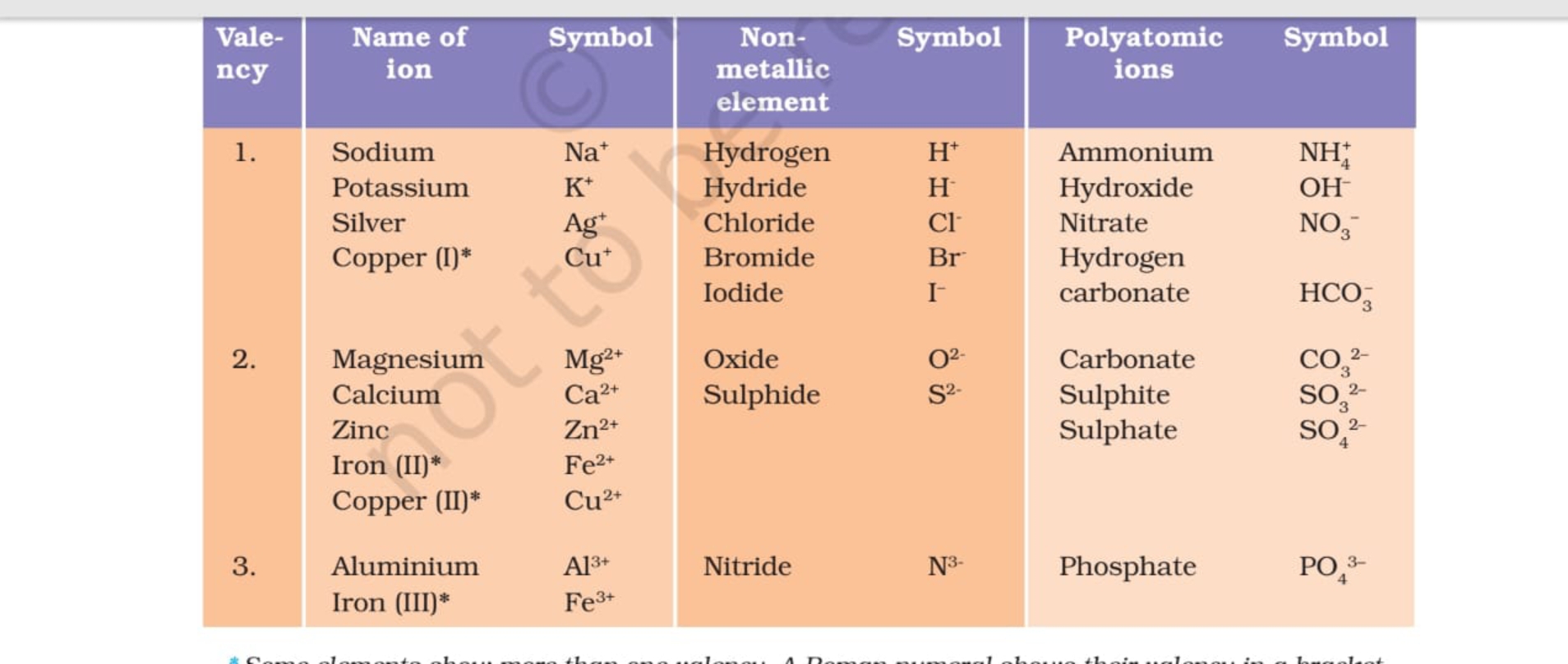
Valency Table Science Notes Teachmint
Structure of atom of Class 9 We have studied earlier about the arrangement of electrons in different shells/orbits. "The electrons present in the outermost shell/orbit of the atom of an element are called valence electrons." In all chemical reactions, only the electrons present in outermost orbit will take part in the reaction.